|
Interim report on two clusters of the novel influenza A (H1N1) infection in Osaka Prefecture, 21th May, 2009
Infectious Disease Surveillance Center, National Institute of Infectious Diseases
Deep appreciation for the cooperation and support to the members and staffs of Osaka City General Hospital, Toyonaka Municipal Hospital, Sakai Municipal Hospital, Osaka City, Osaka Prefecture and public health centers.
Background
The majority of the novel influenza A (H1N1) virus infection reported from Osaka area was consolidated to several schools. As of 20th May, 99 confirmed cases have been reported, of which 64 were from a single private integrating junior and senior high school. Since 18th May, the prefecture changed policy of compulsory hospitalization to the treatment at home for most of the patients based on clinical indication. Here we report clinical information based on profiles obtained from two clusters (previously mentioned private school and nearby elementary school), 69 cases investigated.
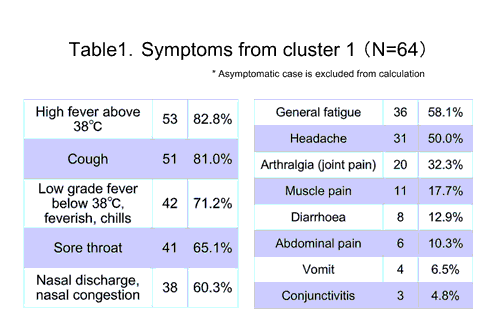
Cluster 1
- setting: integrating junior and senior high school, with 1,934 students and 143 teachers
- study population is 64 confirmed cases ( 59 high school students, 2 junior highs students, 3 teachers), male 49, female 15
- median of age 16 y.o. (range: 13 to 53)
- no oversea travel history reported after April 2009
- 18 hospitalized and 46 medical observation at home
- commute from wide areas ( e.g. Northern Osaka to western Kobe)
- school bus available but crowded
<Course> From 11th May, increased reporting of fever cases observed with focus in the sophomore of high school. Twenty absentees were reported among sophomore on 12th May and then increased to 36 on the next day. Temporary whole grade closure was in place from 13th to 15th following the seasonal influenza procedures. On 16th, samples from influenza A positive students sent off for RT-PCR to public health laboratory and then to National Institute of infectious Diseases given a case confirmation from adjacent Kobe city.
<Data collection> Survey was carried out to 18 hospitalized patients by direct face-to-face interview and to 46 home-quarantined patients on the telephone by school teachers with our technical assistance.
<Symptoms and treatment> Almost all of the cases presented clinical features compatible to seasonal influenza. No case was reported to be under serious condition. In some cases, respiratory symptom and/or vomit preceded high fever by few days.
Antiviral medication was given to all hospitalized cases, oseltamivir to 12 patients and zanamivir to the remained 6. All cases recovered relatively quickly and uneventfully.
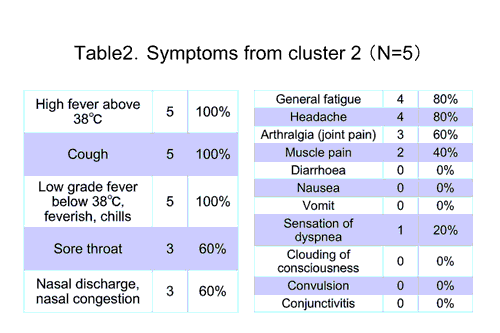
Cluster2
- elementary school, 624 students
- study population is 5 confirmed cases , male 2 , female 3
- age distribution (one 9 y.o., three 11 y.o., one 12 y.o)
- no underline condition noted
<Course> On 16th May, sixth grade female student visited weekend care clinic and diagnosed as influenza A positive by rapid kit test. Zanamivir was prescribed and her ample was sent to regional public health laboratory for further diagnosis. A case was confirmed on 17th and increased to 5 until 19th.
<Symptoms and treatment> Cluster reported from elementary school is still rare in Japan. No case was reported to be under serious condition and recovered relatively quickly and uneventfully after diagnosis. In two cases, respiratory symptom preceded high fever by few days.
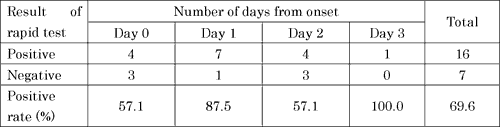
Rapid test results
Sensitivity of the rapid test change by the Day examined, peaked on the Day1 and relatively low on Day0 and Day2. The effectiveness of the rapid test kit is not able to determine by the data presented here because of insufficient information (e.g. type of kit used). However, excluding novel influenza A (H1N1) only by the result of rapid kit test may be premature decision.
Table3.Rapid kit test results of RT-PCR positive confirmed cases of novel influenza A (N1H1)(N=23)
|

